Avantor® offers end-to-end fluid management systems – including Masterflex® peristaltic pumps and aseptic fluid transfer solutions – that support every stage of your biomanufacturing process, at any location worldwide. Our global network of ISO-certified, cGMP manufacturing and distribution facilities across the Americas, Europe and Asia ensures a reliable and uninterrupted supply chain, with access to the most comprehensive library of quality products available.
The unavailability of parts or components isn’t the only type of disruption manufacturers face: If single-use systems are produced but cannot be safely or consistently implemented in cGMP manufacturing environments, then the drug manufacturer can face an impact that is just as — if not more — severe than having delays stem from missing parts.
Partnering with Avantor adds efficiency, consistency and security across all biopharma workflows, so therapies can reach the market, and the patients who need treatments, faster.
Customers turn to Avantor time and again because their unique needs and challenges are solved by our personalized approach to system design. Through upstream and downstream to fill and finish, our fluid handling solutions fit your process and your quality requirements for a world-class customer experience.
LARGEST QUALIFIED PORTFOLIO OF PREMIUM PRODUCTS
Avantor has the largest library of qualified fluid handling components, leveraging industry-wide technologies strategically developed by experts that understand the industry. As an open-architecture manufacturer, Avantor lets you choose from a selection of our own solutions as well as access products from best-in-class manufacturers available from our channel brand, VWR.
Our effective use of vetted, quality single-use products and pumps, coupled with our global footprint, creates agile processes that allow innovation to succeed.
From monoclonal antibodies (mAbs) to cell and gene therapy applications, and from discovery to delivery, we integrate the right products into a fluid handling system that is reliable, repeatable and available on demand.
Single-use supply chain success
Flexible and cost-effective, single-use technologies offer biopharmaceutical companies a way to transform their biologics production and meet the growing demand for these life-changing therapies.

Our global fluid handling capabilities include:
- Fluid handling systems — from transfer assemblies and tubing to bags and pumps — are not one size fits all. We recommend, and work with you to develop, the most ideal system design for your application, including support with prototype and process development.
- Our global fluid handling manufacturing and assembly sites are ISO (International Organization for Standardization) certified and conform to cGMP best practices to help ensure safety, reliability and quality of the products we produce.
- Our Quality Risk Management (QRM) program is a proactive approach to assess, control for, review and correct any potential risk to the quality of our fluid handling assemblies and systems. Our program is applied systemically and with transparency, from the risk-based audits to understand the capabilities of new suppliers to our internal procedures to assess and mitigate risks in our own processes and facilities.
- Our comprehensive supplier management program vets and qualifies first and second sources of key components to ensure a reliable supply chain, globally.
Solving cost and supply challenges in biopharma downstream processing
Learn more about the technologies that therapeutic protein manufacturers and their suppliers can leverage to solve cost and supply challenges in downstream processing.

LOCAL FLUID HANDLING EXPERTS—GLOBALLY AVAILABLE
We collaborate with your team locally to create the right design for your fluid handling system, and then utilize our global manufacturing network to meet your needs efficiently and effectively.
With field-based support on three continents, we meet you and your team where you are, both in your bioproduction process and geographically. This direct access provides the quality fluid handling solutions that best meet the goals, and achieve the outcomes, you have set for your application.
Get the technology choices you’ve come to rely on — from Masterflex pumps and tubing to single-use fluid transfer assemblies and bags — to navigate the challenges, component options and quality and regulatory compliance constraints of the single-use world.
Avantor's global fluid handling network
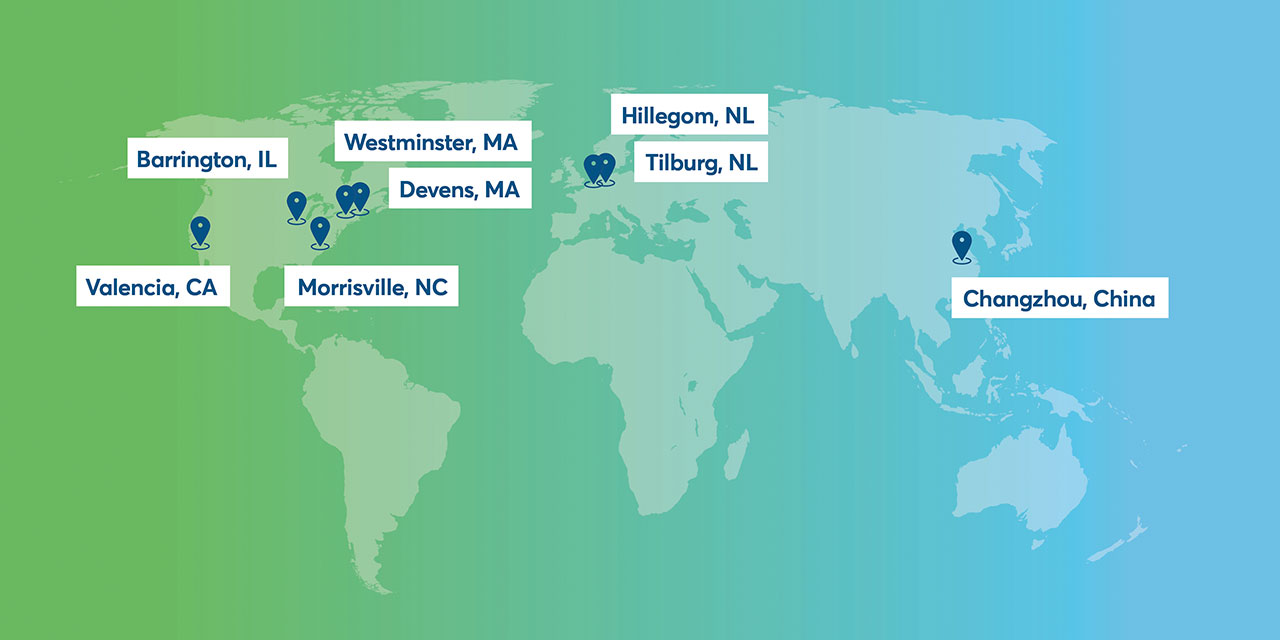
Overview of Avantor’s fluid handling manufacturing footprint
Our fluid handling products are manufactured and assembled in the USA, the European Union and Asia.
- ISO 9001 Certified
- ISO Class 7 cleanroom manufacturing
- cGMP Standards
- Single-use assembly and hose production
- World-class logistics hub for North America
- ISO 9001 Certified
- ISO Class 7 cleanroom manufacturing
- Single-use assemblies production
- ISO 9001 Certified
- ISO 13485 Certified
- Design, develop, manufacture and servicing of active, non-implantable medical components including standard and custom fluid handling equipment and systems including peristaltic pumps.
- Manufacture a range of fluid handing equipment, including fractional horsepower DC motors
- ISO 9001 Certified
- ISO Class 7 cleanroom manufacturing
- cGMP Standards
- Single-use assemblies production
- Center of Excellence for process systems
- Prototype development
- Manufacturing for a range of bioprocessing bags
- ISO Class 7 cleanroom manufacturing
- Single-use assemblies production
Learn more about our fluid-handling solutions
Innovation through customization
We work hand in hand with customers to design personalized solutions that are flexible, scalable and optimized for YOUR needs. Learn how this innovative approach can help you scale up quickly.
Quality and risk mitigation
From sourcing through delivery, we manage risk through meticulous quality assurance and a stable, global supply chain.
