Since the first vaccines were introduced in the late 18th century, vaccines have been critical for medicine and public health. Whether they help eradicate deadly diseases, deal with seasonal illnesses such as the influenza vaccine, or represent the latest developments in vaccine science, Avantor® plays a vital role in research and vaccine production.
Vaccines were developed to target infectious diseases by generating an immune response, such as influenza viruses, and new vaccine concepts—such as mRNA vaccines—focus on immune-oncology applications. In turn, this leads to the growing use of processing applications employing primary cells or cell lines.
In addition to mRNA vaccines, Avantor works with vaccine producers to develop and manufacture other types of vaccines, including:
- DNA vaccines
- Inactivated vaccines
- Non-replicating viral vector vaccines
- Recombinant protein vaccines
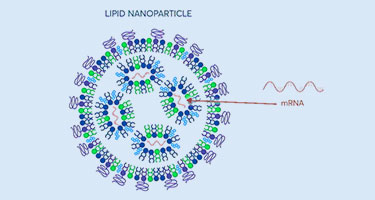
mRNA: Advancing vaccine technology
mRNA uses information in genese to create a blueprint for making proteins. Learn how Avantor supports the mRNA workflow.
Process Development Expertise and Solutions for Every Stage of Vaccine Manufacturing Workflows
Avantor supplies a wide range of cGMP chemicals and excipients, sera, single-use solutions, and other raw materials for vaccine development and manufacturing, including solutions that support COVID-19 vaccines.
We can help you improve your development and manufacturing processes by providing high-quality, ready-to-use, pre-weighed, and pre-packaged cGMP chemicals, including:
- Animal sera
- Excipients
- Fermentation media and supplements
- Growth factors
- Reagents
We combine our product knowledge with our in-depth understanding of how chemicals perform in cell culture systems from discovery through product development, pre-clinical, scale-up, and commercial vaccine manufacturing. That helps you maintain the integrity and activity of vaccine products.
Upstream processing
Avantor can help you optimize vaccine production in mammalian expression and insect systems by providing mammalian cell or insect cell lines that are anchorage-dependent or suspension enabled. Our systems include:
- Culture flasks
- Media components
- Microcarriers for cell lines culture in larger bioreactors
- Roller bottles and roller bottle racks
- Shaker platforms
- Sterile cell factories
We also offer media components that have enriched growth supplements so that you can design your formulations.

Downstream vaccine purification
Vaccine purity, potency, and consistency are critical for concentration and clarification during downstream steps. Our highly stable, low-pH, low salt/high salt, equilibration, elution buffers, and buffer components offer ideal performance. Avantor’s J.T.Baker® brand BAKERBOND™ process chromatography media helps increase product yield by improving the efficiency of purification processes. It also helps enhance selectivity by removing closely related impurities. Avantor can also help optimize the efficiency of your downstream processing steps.

Final fill
Avantor offers the finished packaging vials and stoppers you need to turn a vaccine substance into the final injectable product. Our industry-leading excipient product line—including stabilizers and preservatives—meet your stringent requirements.

Fluid handling solutions
Avantor supports every stage of your vaccine development and manufacturing process with customized fluid handling systems; including single-use technologies and Masterflex® pumps for aseptic fluid handling, storage, and transfer. Single-use systems, such as Adjustable Volume Sampling System (AVSS) and Exact Volume Sampling System (EVSS), precisely collect samples of high-value and/or low-volume products with little to no holdup volume - purging the line easily and avoiding product waste.

Environmental control
Products from industry-leading manufacturers can help you maintain aseptic conditions during the manufacturing and down packing process. Our teams of specialists will use their technical and industry experience to recommend the solutions that best meet your needs.

Explore our products for vaccine manufacturing
Avantor can help you optimize your vaccine manufacturing workflow with innovative solutions to help you achieve the reliability and sustainability you need. Explore our portfolio of cGMP chemicals, single-use solutions, sera, and more on vwr.com.
To find a channel partner in your region, please click here.
Vaccine manufacturing: COVID-19 and beyond
Nandu Deorkar, Vice President of R&D, Biopharma Production at Avantor, shares lessons we can learn from the industry’s historic ramp-up from research to manufacturing.
