Decentralized Clinical Trials
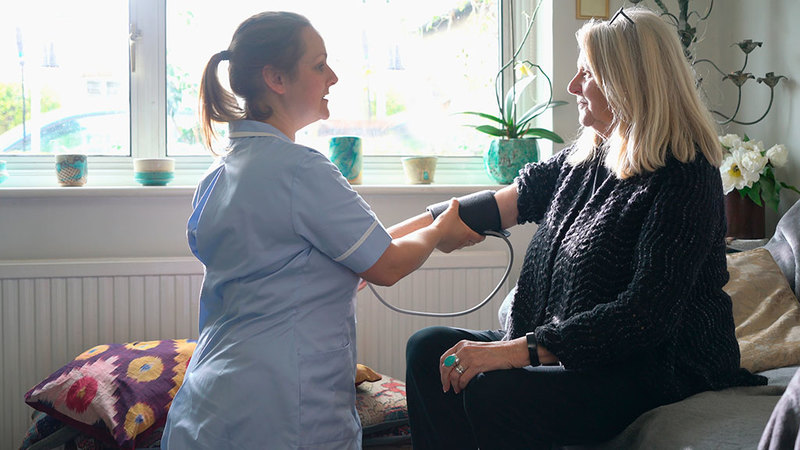
Decentralized Clinical Trials (DCTs) are here to stay; the pandemic has only increased their adoption.
DCTs have many advantages that ultimately combine to accelerate the journey from discovery to delivery, bringing the drug to market faster. Whether a hybrid DCT or fully virtual DCT, there are multiple benefits, but there are also challenges generated by this trial methodology. The supply chain will need to adapt to these challenges to unlock fully the benefits of Decentralized Clinical Trials.
View a presentation from Claudia Berrón, Senior Vice President, Clinical Services to learn more.
Presented by

Claudia Berrón
Senior Vice President, Clinical Services
Claudia Berrón is Avantor’s Senior Vice President, Clinical Services. Prior to this, she was Senior Vice President, Business Development and Commercial Operations for our Biopharma Production business unit. Claudia has over two decades of experience in strategic marketing, covering ideation, value proposition strategies, market segmentation and planning through product and services launch into market. She has been with Avantor since 2015.
Claudia holds an MBA from the University of North Carolina Kenan-Flagler Business School and a bachelor’s degree from Monterrey Institute of Technology in Mexico City.