Inventory management can help streamline operations
Lean inventory management reduces costs
Accelerating Cell and Gene Therapy (CG&T) production
Production process readiness is a key component to successfully accelerating innovation within an increasingly demanding scientific landscape. As your team looks to maximize output, it first relies on materials and workflows to work in unison, in compliance, and as expected. Avantor’s on-site production services’ expertise in process efficiencies, and material and critical environment management, is entrusted to meet the rigorous standards of customers in industrial, biopharma, and pharmaceutical industries. Our chemists, biologists, and engineers adopt your lab’s objectives and deploy customized production support infrastructures fully aligned to your protocols. Seamless collaboration with your team ensures you have just the right production support and materials to create a lab environment primed for accelerating innovation, from discovery to delivery.
A Value Add to your Production Team
For a production site to operate at continuous peak efficiency throughout demand fluctuations, supporting processes such as supply chain and critical environment must be controlled to enable optimized output. Avantor Services helps you at every step, adding value to your production team and ensuring you can deliver the output you want, exactly when needed.
Maximize Efficiencies and Output with Production Support Services
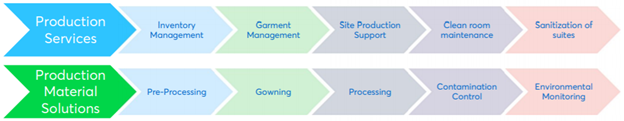
Curating a support system of qualified support teams, optimized processes, and robust digital technologies creates a production environment well prepared to accelerate speed to market. Whether for upstream areas such as cell culture and cell banking, downstream areas such as filtration and chromatography, or key critical environment tasks, Avantor can help. We deploy innovation-enabling production support services for all scientific disciplines utilizing warehouse, bench, or cleanroom environments:
- At scale-up or as part of ongoing optimization, our business process consulting assess lab and warehousing infrastructure, providing production workflow assessments as well as GMP planning, auditing, and relocation.
- Project support: warehouse audits, moves, and planning
- Design stage production consulting
- Process design
- Commissioning projects
- Protocol and standard work development
- Production method and workflow optimization
- Assessments
- Our production material management services spearhead inventory and chemical management throughout the process. Providing 360-degree visibility, Inventory Manager and Chemical Manager software in combination with our established distribution channel VWR, part of Avantor, and ERP integrations, enables demand planning, sourcing, and storing of just the right materials in the just the right amount and location throughout the lab and production process.
- Raw material sampling
- Inventory & chemical management
- Sourcing
- GMP warehousing
- Digital solutions and demand planning
- Chain of custody and material compliance
- Forecasting and demand planning
- Vending
- Production process readiness services manage “at the bench” equipment, materials, and quality control measures to ensure compliance in GMP protocol-driven activities.
- Equipment and instrumentation stewardship
- Production staging and kitting
- Material validation and release management
- Validation and quality testing
- Clinical material receiving and registration
- Protocol driven activities
- Managing the last 3 feet of the supply chain
- Comprehensive critical environment services begin with full-cycle garment management, providing piece-level visibility and tracking across an entire inventory. Cleanroom maintenance and space sanitization, along with testing and monitoring, ensure compliance and quality throughout critical environment work streams.
- Space sanitization
- Garment management
- Testing & monitoring
- Equipment certification and management
- Control and monitoring of aseptic processing cleanrooms
- Glass washing
- Autoclaving
To maximize lab efficiencies and accelerate innovation with on-site production support expertise, contact us.
Explore production services capabilities
Critical environment & sanitization
Maintaining critical environments to meet high standards in an efficient and cost-effective manner requires innovative solutions and expertise.
Production process readiness
Achieving your production goals requires going beyond simply maintaining your critical environments.
Planning & material management
From complex processes and quality controls to loss of manufacturing time, unstable processes risk costly delays, compromising the success of your production activity.
Customer resources & support
Resource library
Your source for insights, brochures, white papers, and success stories.
Contact us
Ready to move your next innovation forward? Avantor Services can help.
