Maximizing clinical trial speed and efficiency
Fast-tracking clinical trials of therapeutic agents and vaccines to counter the coronavirus disease (COVID-19) is a top priority for scientific research companies. As viable vaccine candidates are identified, well-designed clinical trials are absolutely necessary so that governments, healthcare providers and the public trust the vaccines that will be provided.
Once plans for your clinical trial begin, Avantor® is ready to provide a complete set of services and resources to help globally scale your trial, allowing scientists to focus on patient outcomes. Avantor has extensive experience helping to manage the complex logistics and resources needed to set up global, large-scale clinical trials that meet the strictest regulatory and industry standards and help keep your studies on track.
Our experts work with you throughout the process. We develop custom kitting solutions designed specifically for your trial needs, provide equipment and ancillaries that support and optimize your trial and we offer biorepository and archiving services that securely store your samples and data. By taking responsibility for these tasks, we give scientists at research establishments and biopharma companies the ability to focus on the science of designing trials and analyzing results.
To help maximize both the quality and efficiency of the coronavirus vaccine and antiviral therapy clinical trials, Avantor Clinical Services provides:
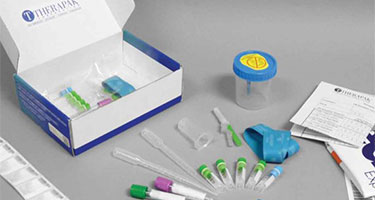
Custom kitting and distribution services: Offering both custom and bulk kitting solutions, we provide over 22 million kits annually to the preclinical, clinical and diagnostic industry. Our experts work with you to design solutions that meet your clinical trial requirements. More than just kitting, we provide global logistic support and management and use state-of-the-art software that can integrate with your systems, enabling real-time tracking, tracing and replenishment.
Learn more through Therapak, our cGMP compliant custom kitting experts
Clinical trial equipment and ancillary services: Having supported more than 2,500 clinical trials in over 80 countries, we have the global expertise and capabilities to manage the full equipment life cycle of your clinical trial. This includes sourcing products and global logistics as well as equipment service, calibration and reporting. The result: We take responsibility to ensure that your clinical trials are properly equipped and remain on time and on budget.
Learn more through MESM, our equipment and ancillary supply experts
Biorepository and archiving services: Our purpose-built storage and archiving facilities can meet your needs throughout the entire clinical trial life cycle. From the minute we take custody of your samples, every step is tracked in real time. Our secure online access can help you manage inventory, submit work requests and generate reports.
Learn more through EPL Archives, our biorepository storage experts