
End of study
Clinical trials don’t end when the last patient leaves. Avantor Clinical Services’ expertise extends from the start of your trial, through to regulatory completion – and we are with you at every milestone along the way. Our end-of-study support ensures you meet vital compliance and good clinical practice requirements, with automatically generated Sunshine Act reporting packs, plus secure equipment and material retrieval, disposal, and storage. With Avantor’s range of clinical trial equipment and ancillary end of study services, our expert care continues as you conclude your trial – so you can focus on your hard-won clinical outcomes.
Meeting key compliance and reporting milestones
Completing your clinical trial successfully requires meticulous end-of-study detailing. Avantor’s Clinical Services team provides three essential components to conclude your study
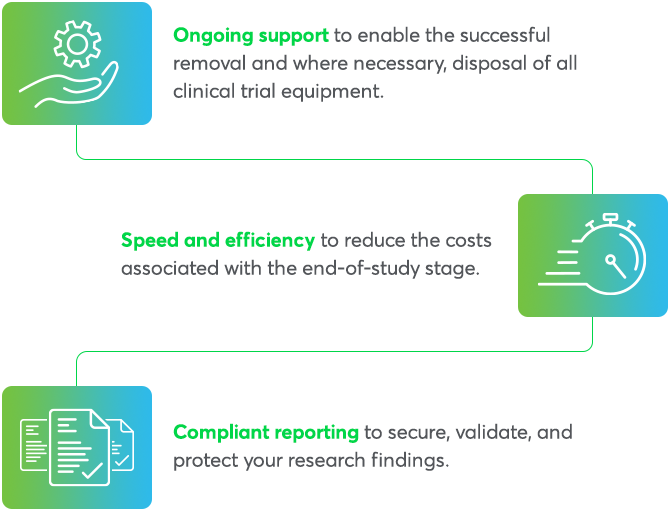
End-of-study services at a glance
Every detail counts, so our end-of-study support provides:
- Sunshine Act reporting packs automatically created for every trial
- Full tracking and traceability of equipment for reporting and Bribery Act compliance
- Comprehensive end-of-study reports
- Logistics solutions to ensure timely collection of equipment
- In-house engineers who can perform decontamination services, recycling, repairing, and disposal of client owned stock
- Storage of your own stock in our controlled warehousing for future use
- Biorepository and archiving of biological samples and research materials
Explore related clinical trial equipment & ancillary service capabilities
To find out more about clinical trial equipment and ancillary sourcing from Avantor Clinical Services, contact our dedicated team today.