Services thought leadership
Decentralized Clinical Trials
How the supply chain will need to adapt to support the growth of Decentralized Clinical Trials

Science of lab management
The value of the lab manager role details how a lab manager can help you move science forward

Maximize your clinical trials budget
Running a clinical trial in 2021 is tougher than ever. The average cost of developing a new drug has reached a staggering $2-3 bn (1) so it’s no wonder that clinical trial researchers are looking for ways to tighten up and make their budgets stretch further.

Why Clinical Trial Equipment Rental Could Transform Your Trial
Buying clinical trial equipment was once the only choice, but not anymore. Could renting the equipment you need be the best choice?

Using social media for clinical trial recruitment
Over the past 10 years, the rapid growth and use of social media networks by the general population has created a powerful new tool for pharmaceutical companies and clinical research organisations (CROs) to engage with patients, and recruit them for clinical trials. CROs and pharmaceutical study sponsors are still learning how to effectively use social and digital media as part of their participant recruitment strategies.

How to avoid costly clinical research delays
A huge majority of clinical trials face delays, costing precious time and money, as well as impacting patients. Here is why managing time, resources and participants are essential in avoiding delays and delivering results.
The importance of logistics and shipping for remote testing and diagnostics
Effective and reliable methods for clinical researchers to conduct patient testing have always been important to the successful execution of studies supporting vaccine development and disease diagnostics.

The rise of home diagnostics in clinical trials
The field of home diagnostics has seen significant innovation and advancement in the past few decades – and in the wake of the COVID-19 pandemic, the growing use of home testing has allowed important clinical research to continue remotely.

10 fundamentals of sample security
The safety and integrity of your research material is of critical importance to your participants, your trial, and your reputation.

Logistics journey of a sample
Clinical trials are essential in bringing new treatments to market, but successful outcomes require global logistics to be carefully considered and rigorously planned.

Focus on quality
Regular monitoring and robust auditing are at the heart of a successful quality control process. They work symbiotically to ensure the quality of your trial data and the protection of your participants...

Partnering to support the lab of the future
The lab of the future is being shaped by the trends of globalization, systemization and digitization.

Building the lab of the future, today
What do we mean, when we talk about the “lab of the future”?

The potential of wearable technology for the future of clinical trial research
The potential of wearable technology for the future of clinical trial research

Meeting today’s changing needs: the vital role of biorepositories and archiving facilities in advancing clinical research
In the ever-changing field of clinical research, valid testing and research outcomes are critical, making reliable storage, archiving, and data retrieval an essential component in the research process.
The rapid growth of clinical trials for COVID-19
The COVID-19 pandemic has been the catalyst for significant shifts in the clinical trial landscape...

Centralized inventory management
Avantor Services provides support so customer can focus on detecting COVID-19 and developing the coronavirus vaccine

Streamlining allocation of critical supplies
Avantor Services leverages its lab & production inventory management expertise to ensure a customer can continue the development of a potential treatment for COVID-19

Multi-site inventory management
Avantor’s expertise in inventory management helps support the development of a potential coronavirus therapy
Direct-to-patient custom kitting solutions
Avantor supports fast-tracking of clinical trials related to COVID-19 disease progression with direct-to-patient custom kitting solution
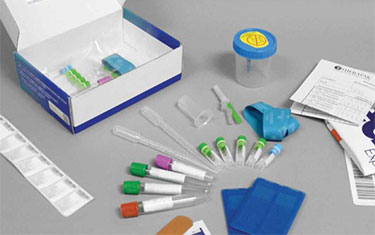
Coronavirus detection
Avantor Clinical Services supports the need to fast-track clinical trials related to COVID-19 disease progression

Success story: Small molecule purification
A global pharmaceutical company sought new ways to help senior scientists focus more time and attention on innovation and strategic drug project work.